Jump to: What Does Magnetic Mean? | Is Pure Silver Magnetic? | Sterling Silver/Silver-Plated Items | Simple Tests | Silver that Seems Magnetic | FAQs
The short answer is no, silver is not magnetic in the usual sense of the word. If a piece of silver jewelry, coin, or other silver item sticks to a magnet, it’s probably not genuine silver.
You might already know that precious metals like gold and silver are often targets of counterfeiting, which makes understanding their basic physical properties essential.
In this article, we’ll explain why real silver isn’t magnetic, how to perform a simple magnet test, and what other metals might cause confusion when checking authenticity.
What Does “Magnetic” Mean?
Magnetism is a natural force, much like gravity, and a physical property found in certain metals such as iron. It arises from the motion of charged particles (electrons within atoms), which generate magnetic fields as they move.
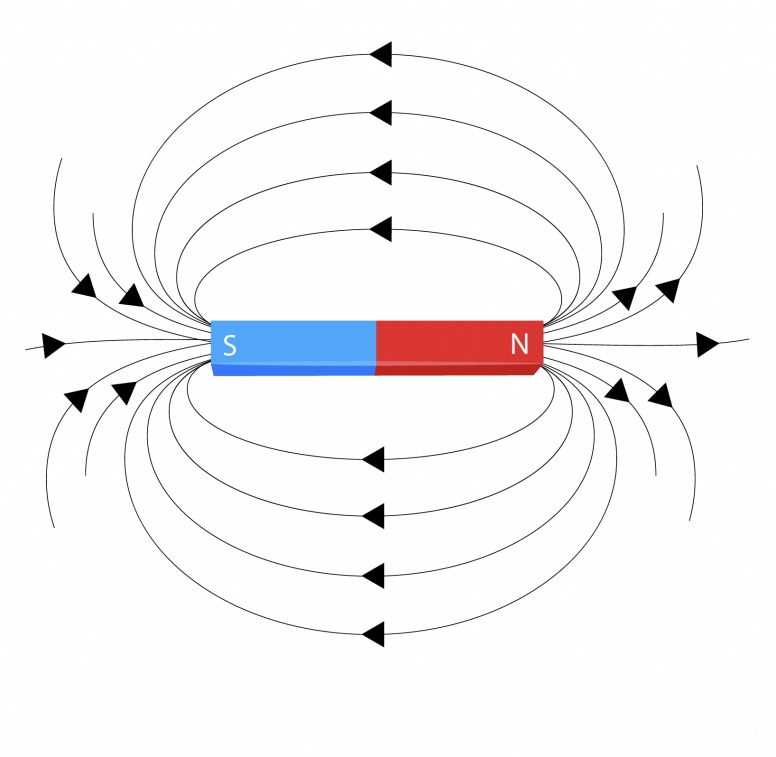
These fields create the effect of attraction and repulsion between objects. Whether a metal is attracted to a magnet helps determine whether it possesses magnetic properties.
So, Is Pure Silver Magnetic?
No, pure silver is not magnetic because it is not attracted to magnets. However, you may come across the term diamagnetic, which describes how silver slightly repels a magnet.
This reaction is based on the way electrons move within the metal’s atomic structure. For example, magnetic metals like iron, nickel, and certain forms of stainless steel behave very differently, since their electrons align to create a stronger magnetic field.
In a diamagnetic material like silver, electrons normally cancel each other out, creating no magnetic field. When an external magnet is near, the electrons shift slightly, generating a weak opposing field. This causes the faint repulsion sometimes observed with silver.
Understanding these differences helps explain why real silver doesn’t stick to a magnet.
What About Sterling Silver, Silver Jewelry & Silver-Plated Items?
You probably asked yourself "is sterling silver magnetic?"
Well, genuine sterling silver is not magnetic because real sterling silver is 92.5% pure silver, with the remaining 7.5% typically copper, a non-magnetic metal.
This is why authentic sterling silver pieces are often marked “.925” or called “925 silver.” In some cases, a necklace clasp or small addition might contain magnetic material, but the main body of the jewelry will not be attracted to a magnet.
If a piece is silver-plated rather than solid, mislabeled, or counterfeit, it may be made from non-sterling metals that react differently.
A Few Quick Ways to Check for Real Silver
Colors
Silver has a bright, white, mirror-like shine that signals its quality. Think of the look of a genuine silver coin: a subtle depth and luster that many lower-quality metals lack.
Interestingly, some high-purity coins can look almost identical; for example, compare the Canadian Maple Leaf struck in .9995 platinum with the one struck in .9999 silver—they can appear very similar at a glance.



However, when polished, real silver may produce a faint black residue from its natural tarnish (silver sulfide). Other metals corrode or discolor differently: copper develops a green patina, iron rusts reddish-brown, and zinc can form a white, powdery corrosion.
Noting these color and tarnish differences is a quick way to help tell genuine silver from other materials (of course, this applies to silver items that have been exposed to air or human contact to some extent, since freshly minted coins or other bullion, like bars, typically show no signs of exposure).
Hallmarks
Even though it may seem obvious, hallmarks are essential for identifying the purity of a silver piece. They indicate the metal’s composition and authenticity.
Before making a purchase, it’s worth checking that the item carries these markings, or better yet, begin your shopping with reputable or popular dealers and jewelers who sell properly stamped pieces.
The standard for silver is sterling silver (925), meaning it’s 92.5% pure silver mixed with copper for strength (this number will probably be on the hallmark, together with the minting company). Any mark above this, such as 950, also qualifies as sterling.
Magnet test
As mentioned before, silver is diamagnetic, which means it’s not attracted to magnets. You can actually test it:
If you hold a silver coin at a 45-degree angle, a moving magnet will slide down slowly rather than stick.
This happens because the moving magnet generates a small electrical field that gently opposes its motion. Try the same test with an ordinary coin and you’ll see the difference; the magnet simply falls off, proving that genuine silver is not magnetic.
At 1:12 of the video, we can see silver repelling the external magnetic field from the neodymium magnet.
The same reaction happens to 24-karat gold at 1:38 of the video. In conclusion, gold and silver are diamagnetic. Gold or silver items will slightly repel an external magnetic field, rather than be attracted by it.
The Gravity Test
The gravity test is one of the most reliable tests you can take at home.
The following video shows a few other noninvasive home silver authenticity tests you can take.
Why Do Some Silver Items Seem Magnetic?
Some silver items may appear magnetic because they’re not pure; they might be silver-plated over magnetic metals or mixed with elements like nickel, iron, or cobalt.
Even a keychain magnet can reveal this: if it sticks, the piece likely contains magnetic material. Pure silver, aluminum, and brass are all non-magnetic and won’t react to a magnet.
When it comes to testing silver, every detail matters.
Final Thoughts
Throughout this article, we aimed to explain why silver isn’t magnetic, how to identify genuine pieces, and what causes confusion with plated or mixed metals.
Simple experiments, like the magnet test or checking hallmarks, can make all the difference. Buying silver from reputable dealers with experience and a solid market reputation can also make the process much easier and more reliable.
We hope these tips have helped you understand the science behind silver and feel more confident spotting the real thing.
FAQs
Does silver stick to a magnet?
As a diamagnetic metal, pure silver does not stick to magnets. Magnetism is a characteristic property of ferromagnetic materials, which suffer strong attraction when close to magnets. If magnets stick to your silver, the item is either fake or an alloy of silver with other metals. Hence, the attraction occurs due to the presence of those metals.
Will a magnet stick to a silver-plated item?
A silver-plated object is not solid silver. It usually has a base metal like copper, brass, or nickel silver, which are non-magnetic or only weakly magnetic. The thin silver layer is diamagnetic and doesn’t attract magnets. If a magnet sticks, the item likely contains a ferromagnetic core, such as steel or iron, which is common in cheaper or mass-produced pieces.
Does a magnet pick up gold or silver?
Pure gold and silver do not hold or stick to a magnet. However, mixed or plated items might. For example, if a ring is gold or silver-plated but contains magnetic metals underneath, a magnet could still attract it. Similarly, if the piece combines gold or silver with magnetic materials, some parts may still respond to a magnet.